
Every electron should have a unique quantum number and quantum states.įrom the Pauli exclusion principle definition, we understood that No two electrons with up spin can be arranged together at the same time no two electrons with down spin can be arranged in a single quantum state. The Pauli exclusion principle states that- no two electrons can have the identical set of quantum numbers or quantum states simultaneously. The Pauli Exclusion principle is one of the important principles for the arrangement of electrons in an atom along with the Aufbau principle and Hund’s rule. Thus the Fermi Dirac distribution follows the Pauli exclusion principle whereas the Bose-Einstein distribution violates the exclusion principle. The Bosons can have or share the same quantum states simultaneously, which violates the exclusion principle.
PAULI EXCLUSION PRINCIPLE IN CHEMISTRY FULL
The Pauli exclusion principle does not hold good for the elementary particles such as bosons that possess full integer spins. The Pauli exclusion principle isn’t only valid for the electrons but also for other elementary particles with half-integral spin, for example, fermions.
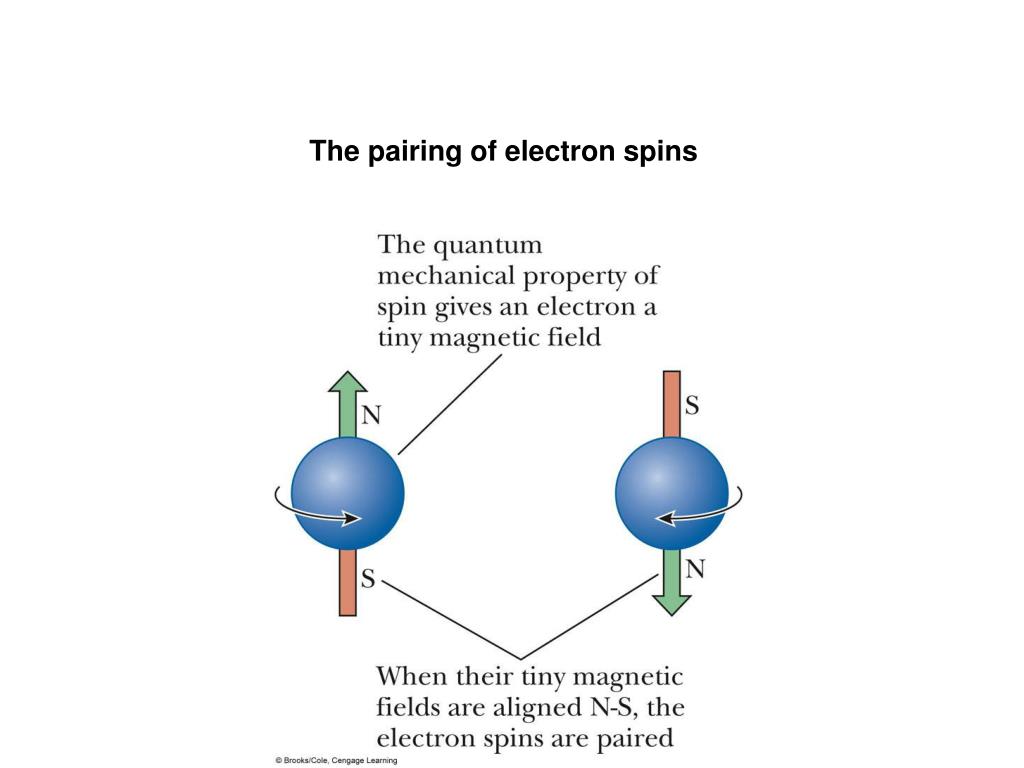
The two electrons occupied in a given quantum state must have an opposite spin or in other words, should be antiparallel to one another. In a given orbital only two electrons can occupy different quantum states. The salient features of the Pauli exclusion principle are as follows: The Pauli principle applies to identical particles with half-integral spin i.e., S = 1/2, 3/2, 5/2 In other words, each electron should have its own singlet state or unique state.

The Pauli exclusion states that no two electrons can have an identical set of quantum numbers. By recognizing that no two electrons can occupy the same quantum state simultaneously, it effectively stops electrons from piling up on top of each other, thus explaining why matter occupies space exclusively for itself and does not allow other objects to pass through it, while at the same time light and radiation are allowed to pass. The Pauli exclusion principle dictates this arrangement effectively forces electrons to take up space in the atom. The exclusion principle is key to the underlying explanations, and that it applies far beyond the realm of quantum physics. This systematic arrangement is related to the number of electrons in a neutral atom, known as the atomic number, denoted by Z. The periodic table of the elements groups elements with identical properties into columns. The physical and chemical properties of elements are directly related to the number of electrons in a given atom.

Pauli’s exclusion principle is one of the important concepts that help us to understand the atomic structures and arrangement of molecules.Īll atoms except hydrogen atoms are multi-electron atoms. The Pauli exclusion principle is valid throughout atomic and quantum physics, it is extremely powerful and broadly applicable. It is known as Pauli’s exclusion principle because it excludes electrons from being in the same quantum state.

Pauli’s exclusion principle states that no two electrons can have the same set of quantum numbers or quantum states. In 1925, the Austrian physicist Wolfgang Pauli proposed Pauli’s exclusion principle.


 0 kommentar(er)
0 kommentar(er)
